Identify, Analyze & Eliminate Recurring Issues with Ease
A CAPA Solution Fully Integrated with Non-Conformance, Quality Management and Manufacturing Execution
In complex manufacturing environments, recurring problems don’t just disrupt workflows—they threaten quality, compliance, and operational efficiency. When systemic issues arise, identifying root causes and implementing corrective actions is essential to maintaining high standards, meeting regulatory requirements, and controlling corrective-related costs before they spiral out of control.
Manufacturo’s Corrective and Preventative Action (CAPA) Management Software delivers a fully integrated, highly customizable solution designed for high-complexity operations. An integrated CAPA system isn’t just important—it’s essential. When CAPA is directly connected to Manufacturing Execution Systems (MES) and Non-Conformance (NC) processes, real-time visibility and data insights empower teams to correct mistakes before they trend out of control. Root cause analysis and corrective actions become streamlined and precise, ensuring not only expedited resolution but also confidence that the right preventive action has been taken. Even in the most demanding manufacturing environments, our CAPA system provides flexibility, transparency, and data-driven insights to implement lasting solutions.

Calculating the Costs of Subpar Quality Management
Recurring quality issues can be financially catastrophic. In industries like aerospace and satellite manufacturing, studies show that the cost of correcting a quality failure can be 100 times the original part cost.
Can you calculate the hidden costs of an inefficient quality system?
Factor in the expenses of rework, approval delays, scrapped materials, and inventory held in Material Review Board (MRB) limbo. These losses often far exceed the investment in a robust quality management system that integrates NC, CAPA, and quality controls across the entire manufacturing process.
But what about the cost of not knowing about quality issues in real time?
Timing is everything. If a trending defect is caught early, the financial and time investment in rework and defect containment can be entirely avoided.
But when quality issues go unnoticed for too long, they cascade into costly failures. How much time is wasted combing through non-conformance and defect records, searching for trends—only to realize there isn’t an effective root cause in place ? How often does a corrective action fail to resolve the issue, leading to layers of quality fixes, increased complexity, rising costs, and still no guarantee that the problem is solved?
A disconnected CAPA system is like relying on a fire alarm instead of a smoke detector—you don’t realize there’s a problem until it’s too late.
Manufacturo is your smoke detector, giving you real-time alerts and insights, ensuring that recurring quality issues become a thing of the past—reducing costs, improving compliance, and safeguarding operational excellence.
CAPA Features That Drive Effective Change Management
Integrated CAPA & Nonconformance Management
- Directly links CAPA projects to nonconformance and quality control processes.
- Supports both minor defects and systemic quality challenges.
- Ensures full traceability from root cause analysis to corrective and preventive actions.
Advanced Investigation & Root Cause Analysis
- Includes industry-proven tools like 5 Whys, Fishbone Diagrams, Fault Tree Analysis, and Pareto Analysis for comprehensive problem-solving.
- Identifies underlying issues across suppliers, production lines, design processes, and audit findings.
- Connects CAPA projects with impact assessments and corrective actions to prevent oversight of critical issues.
Customizable CAPA Workflows & Action Management
- Supports corrective, preventive, and containment actions within a structured workflow.
- Allows custom workflows, approval hierarchies, and conditional-based escalations tailored to your organization.
- Automates process control, such as blocking supplier shipments or restricting work order access until corrective actions are completed.
Compliance-Driven Transaction Control
- Enforces audit and regulatory requirements with built-in tracking and reporting.
- Links corrective actions to supplier performance monitoring, design validation, and manufacturing process improvements.
- Provides clear approval processes to eliminate ambiguity in corrective action tracking.
Scalable for Complex Manufacturing Operations
-
Works across all manufacturing stages, including supplier management, production, quality control, facility maintenance, and shipping.
-
Supports custom action categories to track administrative issues, training gaps, engineering errors, and unexpected shop-floor defects.
-
Integrated with Manufacturo’s broader platform, eliminating the need for disconnected CAPA tracking tools like Jira or stand-alone QMS systems.
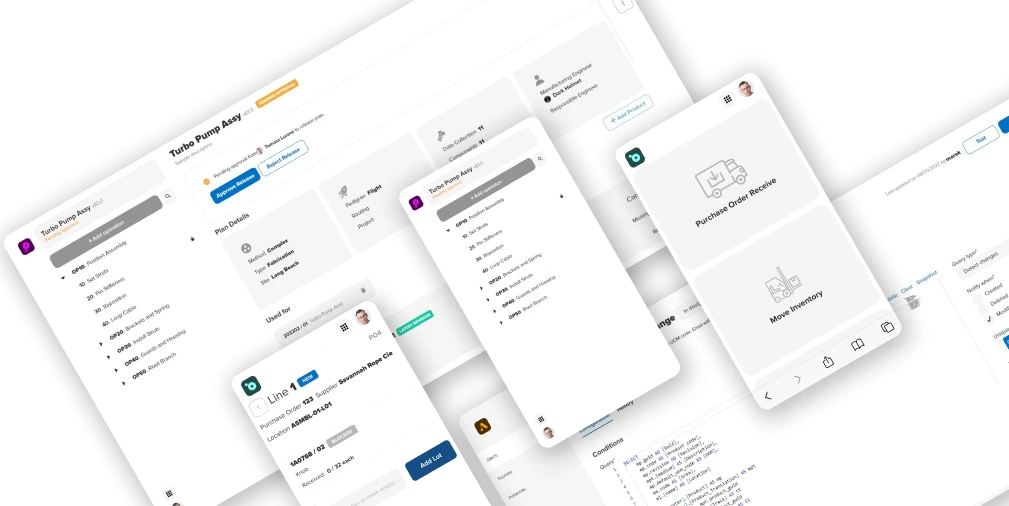
Take a virtual tour to see our software in action
Find out how Manufacturo software can boost your efficiency, quality, and profitability
Why Choose Manufacturo’s CAPA Management?
Eliminates Recurring Issues
Identifies root causes and implements corrective actions to prevent repeat defects.
Ensures Full Traceability
Links CAPA projects to nonconformances, actions, and approvals for a complete audit trail.
Strengthens Compliance
Supports ISO 9001, AS9100, and industry standards with structured workflows and approvals.
Enhances Visibility
Provides real-time tracking of CAPA projects, corrective actions, and supplier performance.
Optimizes Quality Workflows
Automates issue resolution, minimizes production disruptions, and enforces corrective action protocols.
Improves Operational Efficiency
Reduces waste, rework, and downtime by addressing defects before they escalate.
Simplifies Manufacturing Oversight
Seamlessly integrates with nonconformance management, quality control, and transaction tracking.
Replaces Generic Tools
Eliminates reliance on disconnected tracking systems like Jira with a purpose-built manufacturing solution.

Change Management Made Easy
Easily identify and address recurring issues with our CAPA system.Check out our other applications

Seamlessly drive your production across all manufacturing models

Streamline your production processes and cut manufacturing costs

Monitor equipment availability, status, maintenance and calibration dates to eliminate inefficiencies

Ensure the right materials are available to meet production demands.

Control all inventory movements and contain defects at every step

Transforms pull-based replenishment into a connected, responsive system.

Eliminate nonconformance issues, mitigate nonconformity risk, and increase regulatory compliance

Centralize and control all documents within Manufacturo

Integrated, Transparent Action Tracking for the Shop Floor

Gain enhanced traceability to increase safety and compliance
Gain holistic visibility to predict, identify, and eliminate potential issues before they become critical blockers

Embed screens and customize attributes to improve data accuracy, streamline workflows, and increase productivity